- Technology Readiness Level (TRL) was originally developed by NASA, and the TRL framework has evolved into a global standard for evaluating the readiness of the Technology.
- In India, the importance of the TRL framework has grown one step further, becoming a strategic tool for securing the guidance of the regulatory compliance, funding, and helping to build trust with the investors and Partners.
- For the emerging deep-tech startups, especially those building in the complicated world of biotechnology, progress is not just about innovation.
- It’s about reducing risk in a structured, systematic, measurable, and credible way. The Technology Readiness level(TRL) framework has become a foundation tool in guiding the biotech journey.
Why TRL is a Universal Metric?
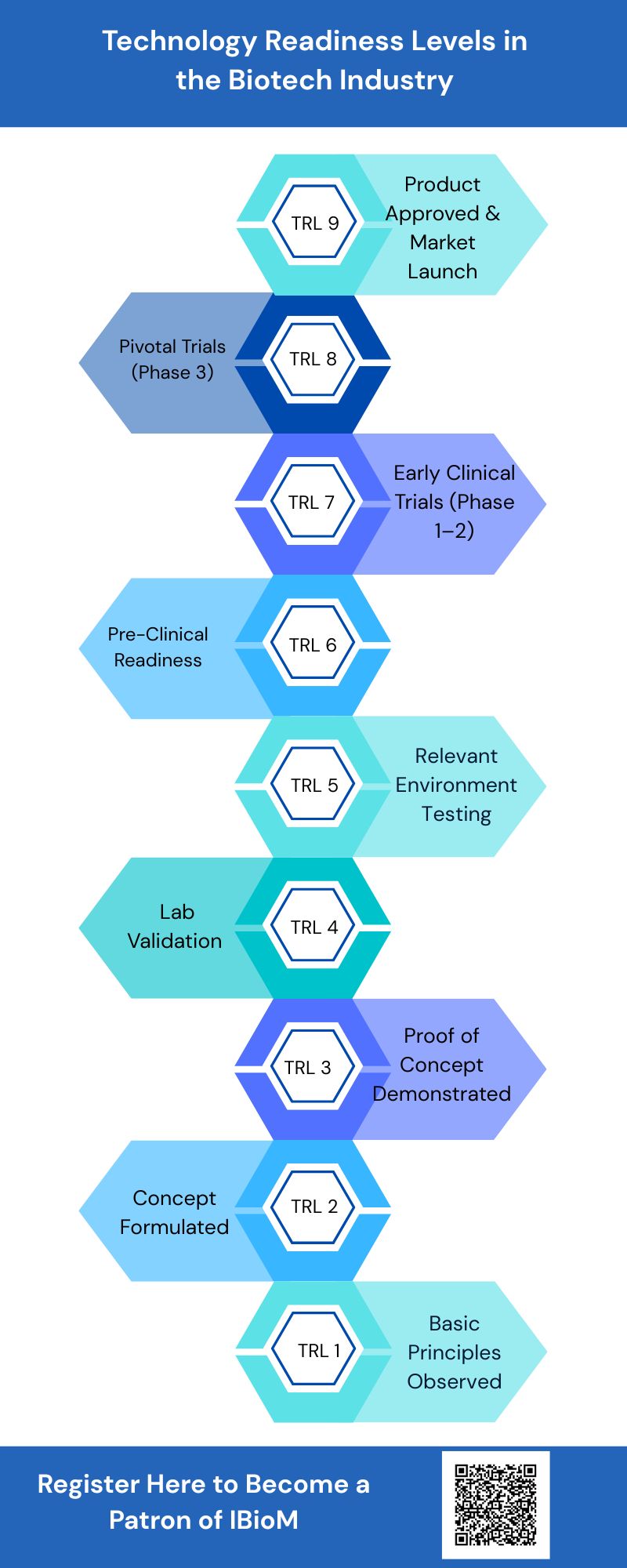
The technology readiness level is a nine-level benchmark that tracks a technology’s journey from a basic scientific idea to a market-level solution. It offers common knowledge that helps the innovators, investors, government agencies, and Industrial stakeholders to evaluate technical risk and readiness.
The nine-level matrix is grouped into three categories.
TRL 1-3: Foundation Research
It includes Proof-of-Concept studies, early scientific exploration, and initial laboratory validation.
TRL 4-6: Development and Validation
Lab-scale prototype development, testing in relevant environments, and initial scaling beyond the lab.
TRL 7-9: Deployment & Commercialization
System-level qualification, regulatory clearance, and full-scale market launch, with each step up the TRL ladder, technical uncertainty directly reduces, strengthening a startup’s valuation and its ability to attract meaningful capital.
India’s Integrated Approach
India has expanded the conventional TRL framework into a more comprehensive evaluation through the TCRM Matrix, used by the policy bodies such as NITI Aayog. The Matrix evaluates innovation on three fronts.
- Technology Readiness Level (TRL): Evaluating the underlying scientific concept has been validated and functions as intended.
- Commercial Readiness Level (CRL): Evaluates the ability to scale, manufacture, and deliver the product reliably and consistently.
- Market Readiness level (MRL): Determines whether a viable and sustainable market exists that is prepared to adopt the innovation.
This comprehensive assessment ensures the support goes not just to strengthen the scientific idea but to emerging innovations that are commercially feasible and well aligned.
Understanding the basics of TRL and Regulatory Pathway in Biotech Industries
In the Biotech Sector, the Technology Readiness level acts as a significant road map to build their product. There are many well-renowned agencies like BIRAC that have adopted TRL descriptors specifically for life science, Biotech, heathtech, and agriculture sectors. These TRL descriptors are well designed and aligned with India’s regulatory compliance checkpoints, making the TRL journey more practical and appropriate.
TRL plays an important role in the Healthcare Industry, which integrates preclinical requirements, ethics committee approvals, and CDSCO clearance. In the agriculture sector, TRLs include the milestones linked to ICAR approval for the field Trials. It is vital to embed regulatory compliance into each stage to prevent late-stage challenges and de-risk their path to market significantly.
Technology Readiness level 1-3:
Proof of Concept & Basic Research taken into account in the first stage TRL.
TRL 1-3, which involves exploring the scientific research idea and showcasing that the idea could work if it is built into a valuable product.
TRL 1: The first and foremost aspect is to understand the underlying scientific innovation and its basic principle.
TRL 2: Once after understanding the innovation and its principle, the technology concept will be framed with preliminary studies, and an application hypothesis will be formed.
TRL 3: In the third level of the TRL, the experiment proof of concept has to be designed with in-lab studies and valid in-vitro data.
Technology Readiness level 4-6:
The 2nd Stage of the TRL plays a crucial role in development & validation. In this phase, the idea starts growing from a lab-scale experiment into more structured testing to test the maturity of the product.
TRL 4: The 4th level involves product optimization, lab-scale validation, and early GMP-lead (Good laboratory practice) safety/efficacy studies.
TRL 5: This level involves developing the scalable manufacturing approaches, testing done in an appropriate environment, and conducting GLP toxicology.
TRL 6: This level involves demonstrating the technology under conditions close to real-time application, including pilot manufacturing and IND (Investigational New Drug application) preparation.
Technology Readiness Level 7-9:
The third stage includes deployment & commercialization, which takes the lab-level innovation forward to real-world testing and final product approval.
TRL 7: This is the most vital level to launch a product in the market, which includes conducting Phase 1 and 2 clinical trials and validating GMP(Good Manufacturing Practice).
TRL 8: To complete Phase 3 trials and prepare major regulatory submissions like NDA(New Drug Application) or BLA(Biologics License Application).
TRL 9: The final level to achieving regulatory approval, launching the product, and monitoring real-world performance.
How TRL works as a Launchpad for Biotech Growth?
On the ground, incubators emphasize and encourage adopting the TRL to bring discipline to life science ventures. While many founders are in their disparate efforts to launch a product, neglecting the structured TRL steps often leads to significant risk in the later period.
For every budding biotech entrepreneur, understanding TRL is non-negotiable. It serves as the foundation that anchors your business and helps you stay resilient during difficult phases. When Technology is combined with commercial and market readiness, it empowers budding startups to scale confidently, reduce risk, and compete globally.
In conclusion, it’s important to understand and be aware of where you are today and where you aim to be in the future, which decides the path of your innovation into impactful, high-value products that are ready for real-world adoption.
We believe that networking with like-minded people is one of the strongest drivers of success. Become a patron of IBioM, our growing community, and be part of this journey.